uNiK™ – Closed production process

Glycostem has translated the natural process of differentiation of blood building stem cells to Natural Killer (NK) cells into a clinical scale and clinical grade NK cell production method. Off-the-shelf umbilical cord blood units are processed within our production platform uNiK™, a completely closed system in a class C clean room environment with own cell expansion technology.
Through uNiK™ we can produce oNKord® for up to 20 patients and viveNK™ for more than 100 patients with one unit of cord blood while maintaining high potency with at least 3 years shelf life.
Safety
Umbilical cord blood is a rich source of stem cells. Collection of cord blood is not associated with risks of donation and thus required ethical limitations of donation for generation of a cell therapy product. Cord blood is available as long as mankind continues to reproduce.
Transfer steps between different disposables for processing devices are performed by sterile welding. Starting material, intermediate stages of the cell product and the final oNKord® cell product are never exposed to a risk of contamination.
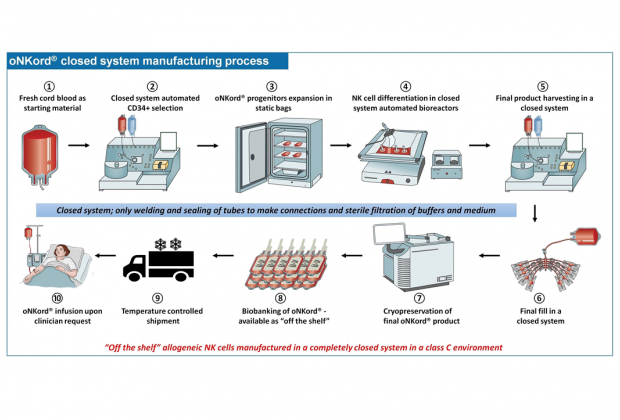
oNKord® production process
1-2: CD34+ hematopoietic stem cells are collected from cord blood units.
3: NK cell progenitor cells are expanded from stem cells within 2 weeks of static culture in a cell culture bag in an incubator; this exposes the cells to two different cytokine cocktails to promote expansion of NK cell progenitor cells over progenitor cells of other cell lineages.
4: Progenitor cells are then transferred to a rocking bioreactor to differentiate into fully functional NK cells and increase their number.
5: NK cells from a culture volume of up to 8 liters are then harvested using a continuous centrifugation process. Cell culture medium is washed out and NK cells are resuspended in an infusion grade solution.
6: Re-suspended NK cells are supplemented with a cryoprotectant and the solution is then filled with fixed cell count, cell concentration and filling volume into cryoconservation bags
7: oNKord® units are cryopreserved using a controlled rate freezer
8: oNKord® units are then banked for use.